miRNA-122 has recently been explored as a biomarker for various hepatic conditions, including drug induced liver injury (DILI) as well as for hepatitis, non-alcoholic liver disease/non-alcoholic steatosis (NAFLD/NASH) and liver cancer. A change in levels of miRNA-122 in the blood has been reliably confirmed as an indicator of liver injury. In acute situation, this change is observed before the increase in amino-transferase activity, making it an early indicator of liver disease. The assay works with cell based culture, animal models, with patients and companion animals such as dogs.
DESTINA offers a complete miRNA-122 profiling service combining its ChemiRNA™ Tech with the highly sensitive Luminex® systems (MAGPIX®, FLEXMAP 3D®). Cell supernatants and lysates, plasma and serum samples can be prepared and delivered using DESTINA Stabiltech buffer without the need for expensive refrigerated transport. miRNA-122 can then be detected and quantified directly from these biological samples without the need for extraction, reverse transcription or amplification using standard Luminex system workflows.
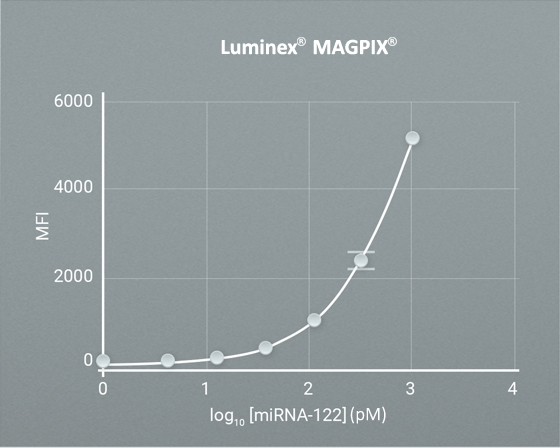
Direct detection of synthetic miRNA-122 spiked into commercially available serum with LoDs of sub-pM level.
In our state-of-the-art laboratory, our expert scientists can perform the miRNA-122 profiling related to liver conditions.
-
-
- Quantifying levels of miRNA-122 in blood plasma and serum samples of patients with:
- viral-, alcohol- and chemical-induced liver injury.
- Patients with organ rejection after liver transplantations.
- Patients affected by hepatitis C.
- Hepatectomy-induced liver injury in patients with hepatocellular carcinoma.
- Laboratory test animals used in drug toxicity testing.
- Cell based reactor systems for drug toxicity testing.
- Quantifying levels of miRNA-122 in blood plasma and serum samples of patients with:
-
Over the years, DESTINA has validated its method using patient biological samples (Case study 1). Additionally, DESTINA has carried out studies in collaboration with leading pharmaceutical companies and CRO using animal models and primary hepatocytes with very small sample sizes (Case study 2).
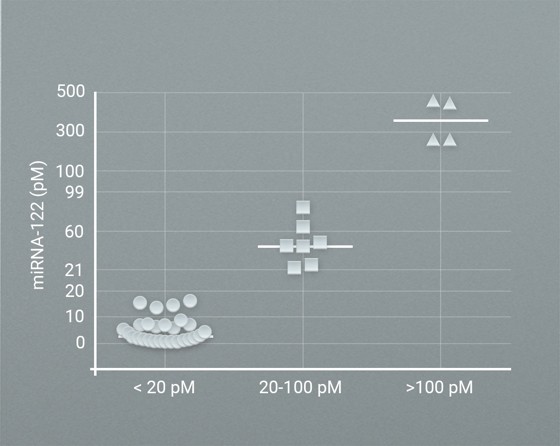
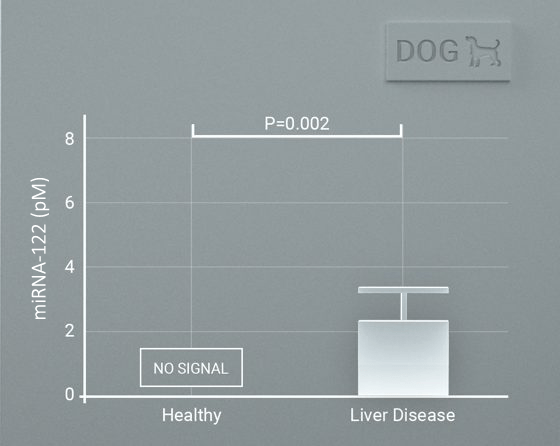
Case study 1
Profiling of miRNA-122 levels in 192 patients with: a) acute liver injury, following acetaminophen/paracetamol overdose; b) patients with non-alcoholic fatty liver disease (NAFLD); c) patients with Hepatitis B.
Case study 2
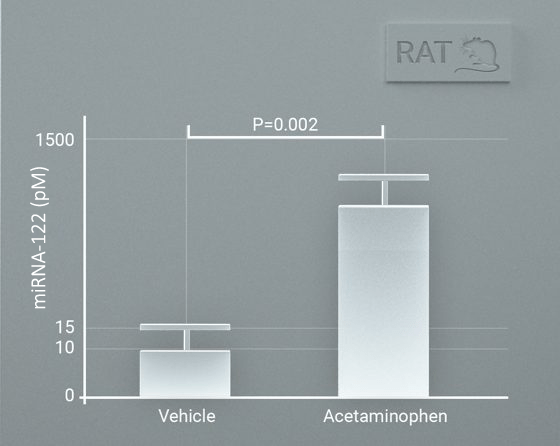
miRNA-122 profiling in rats and dogs. Rats were treated with acetaminophen with a miRNA-122 increasing compared with vehicle treated rats. miRNA-122 was increased in dogs with clinical liver disease compared with healthy dogs. Data were generated using very small sample volumes (2-10 uL).
Develop your own Assay
Our Staff can upgrade your actual miRNA profiling into reliable, direct and absolute quantification of your target miRNA using ChemiRNA™ Tech. DESTINA works with its customers to develop customised DGL-Probes in order to profile the required miRNA biomarkers.
Challenging samples like serum, plasma, saliva and urine can all be handled using ChemiRNA™ Tech. The current limitations and challenges to accurately and reliably analyse for miRNA biomarkers (losses in preparation, isomiRs, and endogenous matrix inhibitors) simply do not enter the equation when using the ChemiRNA™ Tech.
Depending on your needs, DESTINA can provide a full service through to sample testing, or a licensed supply and tech transfer to your laboratories. We provide solutions for quantitative analysis of miRNAs in cells, tissues, extracellular vesicles and biofluids.
How to create your own ChemiRNA™ Tech assay: