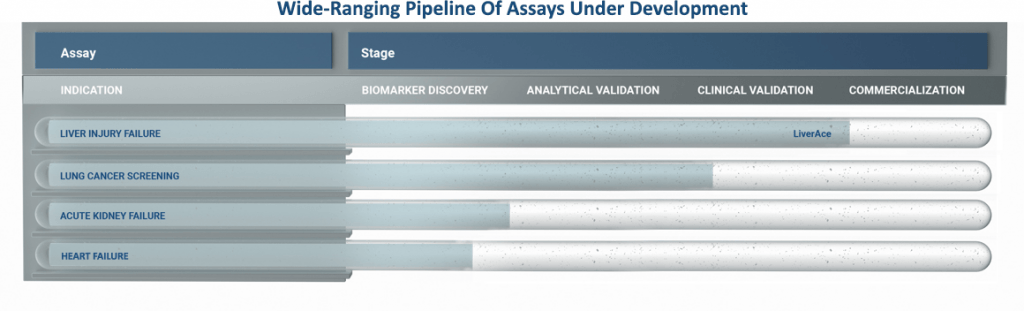
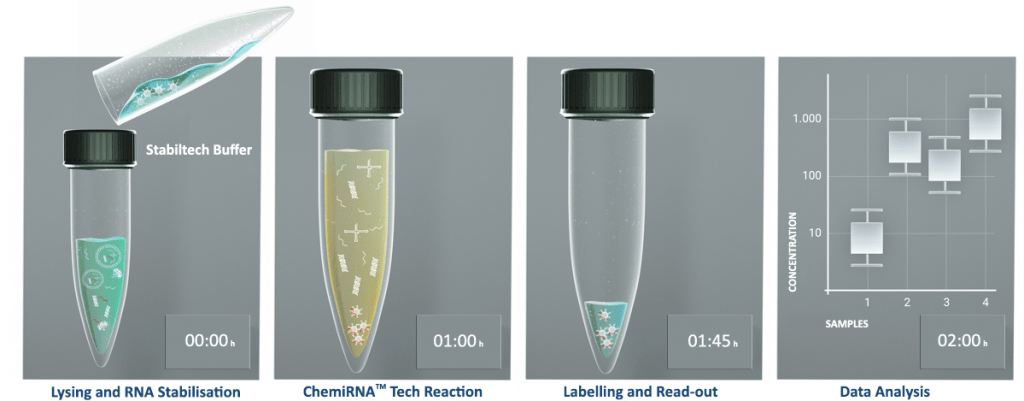
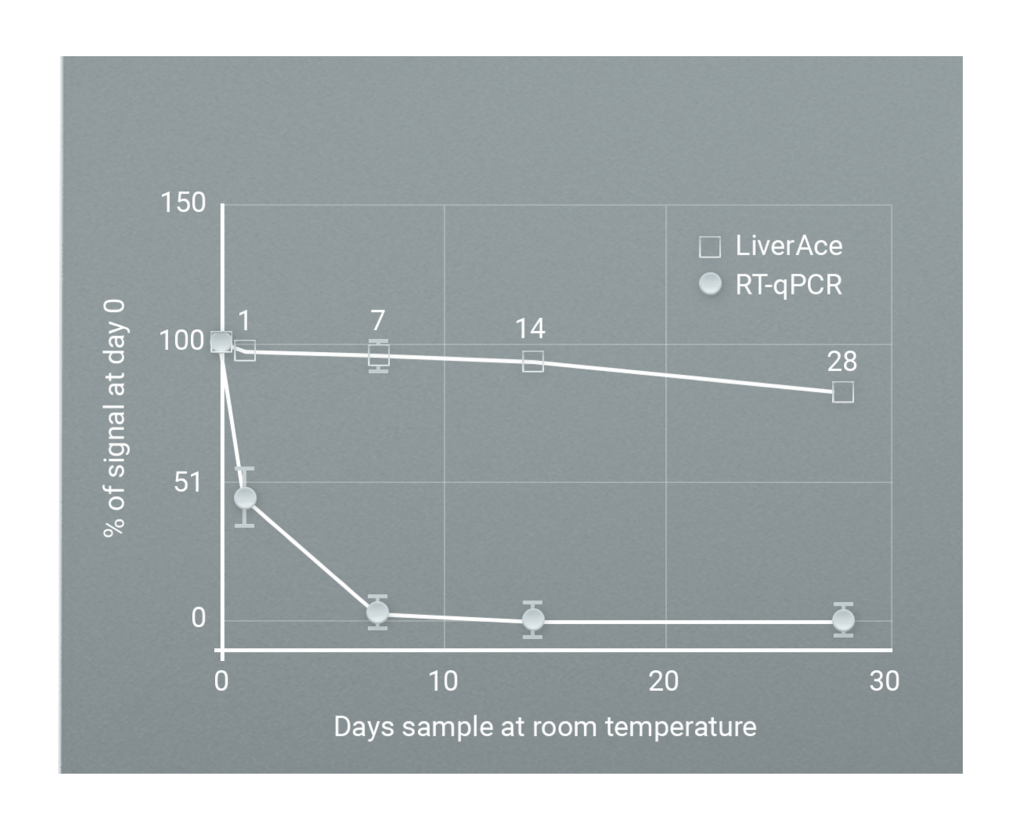
DESTINA is focussing its Assay and kit development activities in disease areas where:
-
-
- There is a large opportunity in both pharmaceutical development and clinical diagnostics.
- There is currently no accurate and effective diagnostic.
- Where miRNAs represent an opportunity to provide earlier detection and improved assay specificity.
-